 |
 |
 |
 Health & Beauty | August 2006 Health & Beauty | August 2006  
New Method Makes Embryo-Safe Stem Cells
 Matt Crenson - Associated Press Matt Crenson - Associated Press

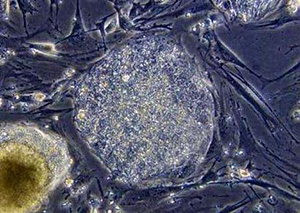
| | A microscopic view of undifferentiated human embryonic stem cells. A Massachusetts company said on Wednesday it had developed a way to make human embryonic stem cells without harming the original embryo, a finding it said could dispel ethical objections to promising medical research using such cells. (Reuters/University of Wisconsin) |
In an innovative move, a biotech company has found a new way of making stem cells without destroying embryos, touting it as a way to defuse one of the country's fiercest political and ethical debates.

Some opponents of the research said the method still doesn't satisfy their objections and many stem cell scientists and their supporters called it inefficient and politically wrong-headed.

But a spokeswoman for President Bush, who vetoed legislation last month that would have allowed federal funding for embryonic stem cell research, called it a step in the right direction.

And Robert Lanza, an executive with Advanced Cell Technology, which created the new stem cell lines, said: "This will make it far more difficult to oppose this research."

Stem cells have become a Holy Grail for advocates of patients with a wide variety of illnesses because of the cells' potential to transform into any type of human tissue, perhaps leading to new treatments. But the Vatican, President Bush and others have argued that the promise of stem cells should not be realized at the expense of human life, even in its most nascent stages.

The new method works by taking an embryo at a very early stage of development and removing a single cell, which can be coaxed into spawning an embryonic stem cell line. With only one cell removed, the rest of the embryo retains its full potential for development.

The method was described online Wednesday in the British journal Nature. The journal published a similar paper by Advanced Cell Technology last year demonstrating the technique's viability in mice.

"The science is interesting and important," said John Harris, a professor of bioethics at the University of Manchester in Great Britain, commenting on the biotech company's efforts.

But few believe it will resolve the bitter ethical battle over stem cell research.

"This will please no one," predicted a longtime critic of the company, Glenn McGee, director of the Alden March Bioethics Institute in Albany, N.Y.

Some stem cell researchers complain that the new approach, though it may hold future promise, simply isn't as efficient as their current method of creating stem cells. That procedure involves the destruction of embryos after about five days of development, when they consist of about 100 cells.

Meanwhile, hard-line opponents of stem cell science argue that the technique solves nothing, because even the single cell removed by the new approach could theoretically grow into a full-fledged human. Some also object over the possibility the procedure could harm the embryo in an unknown way.

The method "raises more ethical questions than it answers," said Richard Doerflinger of the US Conference of Catholic Bishops.

US law currently bans federal funding of any research that harms human embryos. A White House spokeswoman said the method's eligibility for funding could not yet be determined, "but it is encouraging to see scientists at least making serious efforts to move away from research that involves the destruction of embryos."

President Bush has said that he personally opposes any research that sacrifices embryonic life, even to save an existing person. In August 2001 the president limited federal funding to research on a few dozen stem cell lines that had been created up to that point.

Scientists complain that the decree has severely crippled progress in the field. But recent developments have moved them toward their twin goals of attracting non-federal money for stem cell research and overturning the restrictions.

Several states, including California, New Jersey and Illinois, have set up ways to fund the research. A number of Democratic candidates in this year's congressional elections are focusing on the issue.

The research at Advanced Cell Technology subverts those efforts, McGee said. But writing in Nature earlier this year about the demonstration of the technique in mice, Stanford University stem cell researcher Irving Weissman disagreed.

"Although the efforts cited here will be criticized as a diversion of good science by politics, I believe all of these attempts to advance and translate medical science should be pursued in parallel," Weissman wrote.

Scientists at Advanced Cell, based in Alameda, Calif., devised a clever means of piggybacking on existing fertility treatments to avoid the creation, manipulation or destruction of embryos specifically for the production of stem cells. The fertility procedure, known as preimplantation genetic diagnosis, or PGD, is used when parents want to avoid having a child with a lethal or severely debilitating birth defect. About 1,000 such procedures are performed each year in the United States.

PGD begins with in vitro fertilization to produce numerous embryos. At a very early stage of development, when the embryos are no more than a ball of eight to 10 cells, a technician extracts a single cell from each one. The extracted cells are tested for genetic disorders, and those free of defect are then implanted in the mother in the hope they will develop.

The new stem cell production method takes a cell extracted during PGD and allows it to divide. One of the two resulting cells is genetically tested as in normal PGD; the other is cultured to encourage the development of stem cells.

"It's nothing revolutionary," said Yury Verlinsky, a Chicago physician who specializes in PGD.

Though the new procedure may satisfy the president's objections to stem cell research, it does not meet the ethical standards of the Roman Catholic church, which opposes both PGD and in vitro fertilization.

Advanced Cell Technology was able to produce two viable stem cell lines from a total of 16 embryos. The lines appeared to exhibit the full potential of embryonic stem cells to develop into any type of human tissue, the researchers reported, but additional study is needed to verify that.

"I think this will become a standard way of producing stem cell lines," said Ronald M. Green, a Dartmouth College professor of religion who is an unpaid bioethics adviser to Advanced Cell Technology.

The company, which has been struggling financially, owns about 300 patents that it hopes to develop into medical treatments. After news of its announcement broke on Wednesday, the price of its over-the-counter stock shot up from 42 cents to more than $1.70 per share. | 
 | |
 |



