
|  |  |  Health & Beauty Health & Beauty  
Herbal Supplements Face New Scrutiny
 Laura Landro - WSJ.con Laura Landro - WSJ.con
go to original
September 15, 2010


| | Cranberry (extracts, tablet, capsules) May prevent urinary tract disorders, stomach ulcers, dental plaque; anti-cancer benefits. (Getty) |  |
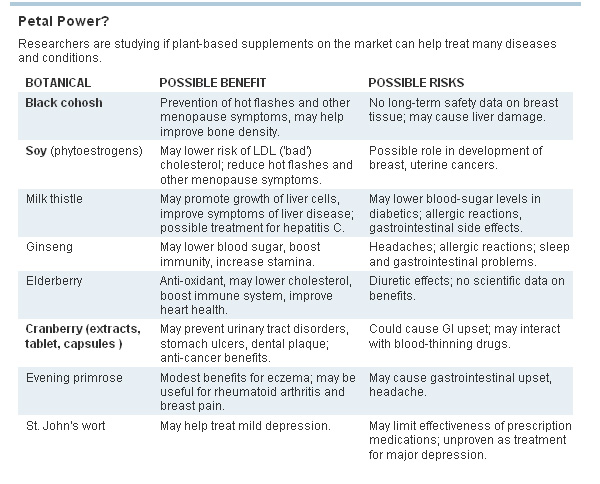
Elderberry extract and acai to boost the immune system. Black cohosh to lessen the discomforts of menopause. Soy capsules to prevent bone loss and prostate cancer.

Many botanical supplements - made from the seeds, bark, leaves, flowers and stems of a wide range of plants - have been widely used as folk remedies for centuries. Americans have been consuming growing quantities of the supplements in hopes of warding off disease and easing symptoms of various conditions. But there is scant scientific evidence to support their health benefits.

Now, the federal government is stepping up research into the safety and effectiveness of a wide range of over-the-counter supplements, including plant oils, garlic, soy, elderberry, licorice, black cohosh, St. John's wort and the Asian herb dong quai. The aim is to better understand how compounds in the plants affect health and to help consumers make more informed choices about supplements, which can interact with prescription drugs, cause side effects or lead to new health risks. Sales of botanical supplements in the U.S. topped $5 billion last year, up 17% from five years earlier, according to the non-profit American Botanical Council.

"Sometimes people assume because a product is natural, it is also safer. But these compounds can have both benefits and potential side effects and we need to understand both of those," says Floyd Chilton III, director of the Center for Botanical Lipids and Inflammatory Disease Prevention at Wake Forest University Baptist Medical Center in Winston-Salem, N.C. Dr. Chilton's center received a $7.5 million federal grant to study botanicals, including whether plant oils such as echium and borage can help play a role in preventing cardiovascular disease, asthma and diabetes.

"People are using supplements for purposes for which they were not intended," such as treating health conditions they have self-diagnosed, or using multiple supplements in combination with prescription medications, says Marguerite Klein, director of the Botanical Centers Research program at the National Institutes of Health. One concern, she says is the heavy use by women of black cohosh to treat menopause symptoms, such as hot flashes. Limited research seems to support the black cohosh's benefit. But it isn't known how the botanical works. Black cohosh has been linked in some patients to liver damage, and breast-cancer patients are often advised to avoid using it because its effects on breast tissue are unknown.

Helping to spur the research initiative are the Office of Dietary Supplements and the National Center for Complementary and Alternative Medicine, both part of the National Institutes of Health. The agencies last month awarded grants totaling about $37 million to five dietary supplement research centers, expanding a program that has already awarded more than $250 million in research grants for herbs and botanicals since 2002. The NIH is also funding research into botanical products through the National Cancer Institute, which is interested in how components in botanicals might influence cancer risk and tumor growth.

Studies funded by the federal grants have so far shown that chamomile capsules may help reduce anxiety compared to a placebo and that an extract from the milk thistle plant can interfere with the life cycle of the hepatitis C virus. They also have refuted some purported benefits of botanicals, showing, for instance, that ginkgo biloba does not prevent heart attack, stroke, or cancer, or stem memory loss and that St. John's wort was no better than a placebo in treating symptoms of attention deficit hyperactivity disorder in children and teens.

Unlike drugs, which must be tested in clinical trials and approved by the Food and Drug Administration before they can be marketed, botanicals and other supplements don't require regulatory approval. The FDA in June began requiring all supplement makers to follow strict quality manufacturing standards, but the agency only periodically inspects plants.

An investigation published in May by the General Accounting Office found deceptive marketing practices at a number of online retailers, including claims that supplements could prevent or cure conditions such as diabetes, cancer, or cardiovascular disease. The investigation also found trace amounts of potentially hazardous contaminants, such as lead or bacteria, in 37 of 40 herbal dietary supplement products it tested.

Tod Cooperman, president of ConsumerLab.com, which tests supplement brands for quality, says the group finds problems with about 25% of all supplements, and especially with herbal products, many with ingredients from overseas. A recent review of supplements made from ginseng - commonly taken to boost energy and vitality - found that 45% failed quality tests because they didn't contain the advertised amount of ginseng or were contaminated with lead. Test results and other information are available to members, who pay $30 annually.

Consumers also can find information about potential uses, benefits and risks of dietary supplements at federal websites ods.gov and nccam.gov. Another government site, Medlineplus.gov, grades scientific evidence on a variety of supplements.

William Cefalu, director of the Pennington Biomedical Research Center at Louisiana State University in Baton Rouge, says researchers are only beginning to understand how thousands of different compounds in a single plant may interact, and how the concentration of a particular plant chemical affects its potency. For example, peppermint tea is considered safe to drink, but peppermint oil, often taken for irritable bowel syndrome or indigestion, is much more concentrated and can be toxic if used in high doses.

Because the potency of wild plants can vary, some researchers are cultivating their own. At the Center for Botanical Interaction Studies at the University of Missouri in Columbia, 600 types of soybean seeds are being cultivated to study different concentrations of the same compounds in the plants and how they might work to prevent prostate cancer. The center is also growing 60 types of elderberries to study the plant's possible role in boosting the immune system against infection and fighting cancer and inflammation in the body. Center director Dennis Lubahn says there may be variations in individual plants that will make a difference in how well they fight disease. "We've come a long way from the traditional medicine woman sampling leaves in the forest," he says.


informedpatient(at)wsj.com |

 |
|  |



